Your immune system can kill you.
We’re trying to understand why it usually doesn’t.
Area 1: Autophagy in macrophage activation and cytokine storm syndrome (CSS)
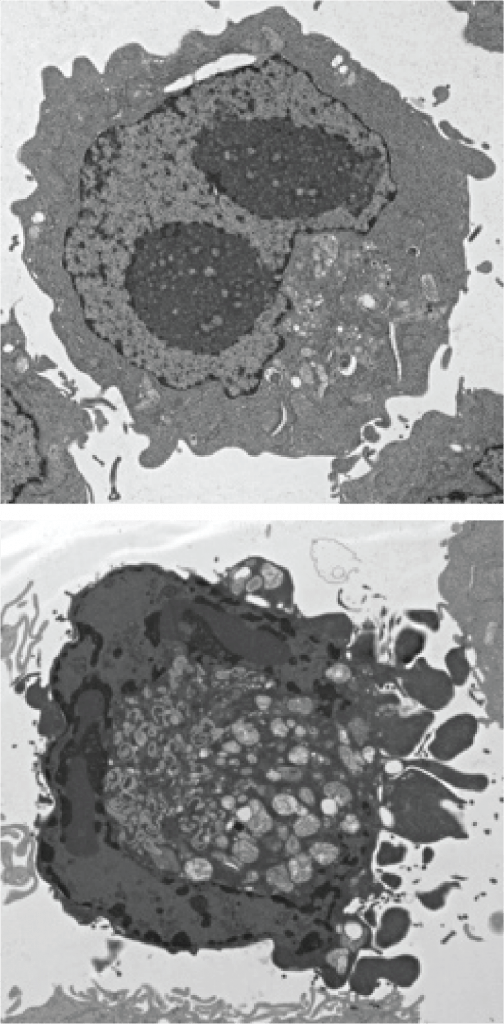
Autophagy is a highly evolutionarily conserved pathway in which cytosolic components are enveloped in a double-membrane vesicle and delivered to the lysosome for degradation. This activity regulates diverse aspects of host immunity. Among these emerging roles, we found that autophagy genes in myeloid cells protect against interferon gamma (IFNγ)-induced cell death and fatal disease in a murine model of tumor necrosis factor (TNF)-induced Cytokine Storm Syndrome (CSS) [PMID: 31346084]. The protective role of autophagy limited a Receptor Interacting Kinase 1 (Ripk1)/Caspase-8-mediated apoptotic program in macrophages. We are actively pursuing a number of important questions related to this area, including: What is the precise role of macrophage cell death and survival in CSS in vivo? In which myeloid cell type(s) does autophagy protect against CSS? If the canonical lysosomal degradative capacity of autophagy is required for its protective effects, what are the cellular targets?
Area 2: Immunometabolism in macrophage activation
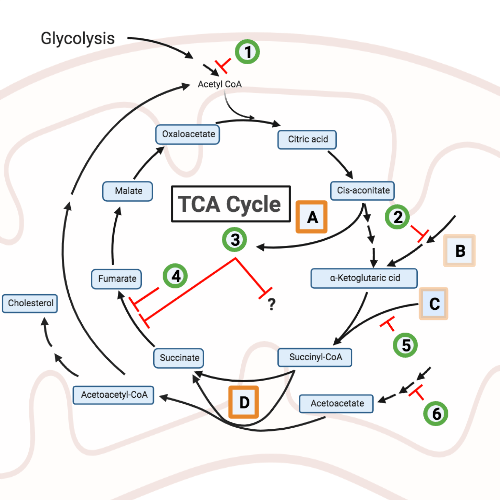
Classical proinflammatory macrophage activation by IFNγ and LPS results in a shift from oxidative to glycolytic metabolism through multiple well-described mechanisms. However, our understanding of the relative role of these bioenergetic shifts and/or activities of specific metabolites in macrophage responses and disease states remains incomplete. In published [see PMID: 31346084] and in unpublished work, we found an important role for genes involved in the TCA metabolic cycle in mediating macrophage survival and cell death. We are also studying the role of nucleic acid metabolism and the role of cytosolic nucleic acid sensing in macrophage activation. In unpublished work, we have found an important role for a regulator of cytosolic nucleic acid accumulation in resistance to viral infection, and additional novel factors involved in cytosolic nucleic acid-induced cell death. We are utilizing genetic and pharmacologic approaches to define the metabolites and pathways that mediate immune activation of macrophages, and the role of endogenous triggers of inflammation. Opportunities exist to explore this area using metabolomics, targeted and unbiased chemical/pooled genetic suppressor screening, and small molecule screens to identify tool compounds and drug candidates.
Area 3: Cytokine storm due to viral infections including SARS-CoV-2
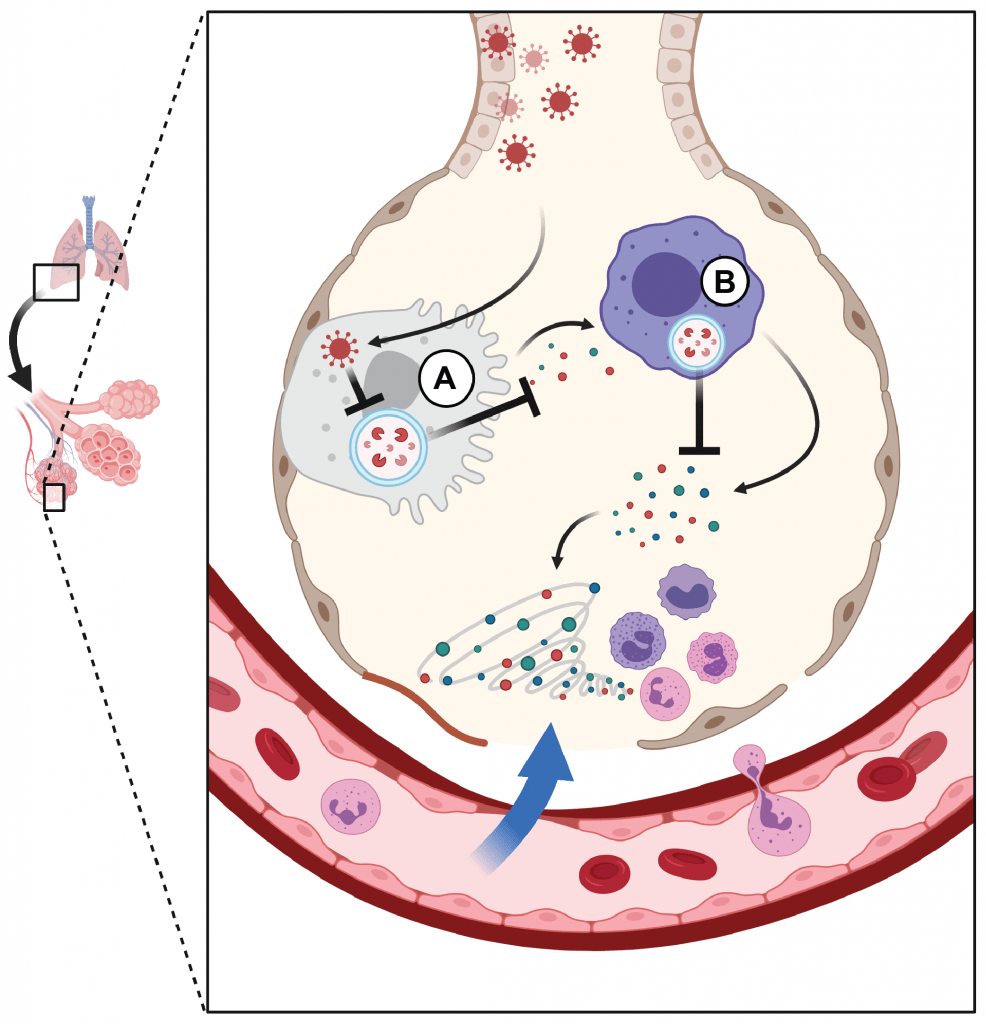
The emergence of SARS-CoV-2, the etiologic agent of COVID-19 disease, has highlighted the threat of novel infectious diseases to humans for which no pre-existing therapies exist. COVID-19 is characterized by a dysregulated immune response in which tissue injury occurs via unclear mechanisms, raising the possibility that host-directed immunomodulatory therapies could show benefit. Since autophagy plays an important role in immune responses (including to IFNγ and TNF, which have been implicated in severe COVID-19), we hypothesize that the pathway may modulate the host response to SARS-CoV-2. Additionally, factors encoded by coronaviruses from multiple lineages have been shown to rearrange host autophagy-associated membranes. We are developing novel approaches to better understand the role of autophagy and host pathogen interactions at the interface of infected respiratory epithelium and surrounding alveolar macrophages.